Currently, the standard method of radical treatment of brain diseases is surgical intervention to remove certain areas of the brain (e.g., tumors), which involves opening the cranium and further incision through healthy tissue on the way to the target area. Such intervention is associated with the risk of bleeding, infection and other postoperative complications, and the outcome of the operation largely depends on the size of the target area and its location in the brain.
Minimally invasive interventions known in neurosurgery, such as endoscopic neurosurgery, cryoablation and radiofrequency ablation, can reduce the size of the skull opening and shorten the postoperative recovery period, but still require access through an opening in the skull and may be accompanied by damage to healthy brain tissue to reach the target area. Radiation therapy is also not a non-invasive procedure, since it uses ionizing radiation, which has a destructive effect on healthy brain tissue as well.
Recently actively being developed methods of non-invasive surgery using high-intensity focused ultrasound (HIFU) provide the possibility of invisionless fractionation of specified areas of the brain by ultrasonic heating of tissue up to the temperature of thermal necrosis under the control of magnetic resonance (MR) thermography. Thermal HIFU technology is used in clinics in Russia and other countries to treat movement disorders (essential tremor, tremor in Parkinson’s disease, dystonia, etc.) by producing localized thermal lesion inside the brain. However, to date, the accessible area of the brain for thermal HIFU surgery is limited to the central parts of the brain – thalamus and globus pallidus – due to the risk of overheating the bones of the skull, which effectively absorb ultrasound with shallow focusing. Other limiting factors are heat diffusion and blood flow, which reduce the accuracy of the exposure, as well as the need for the expensive MR tomography devices to control the temperature during surgery.
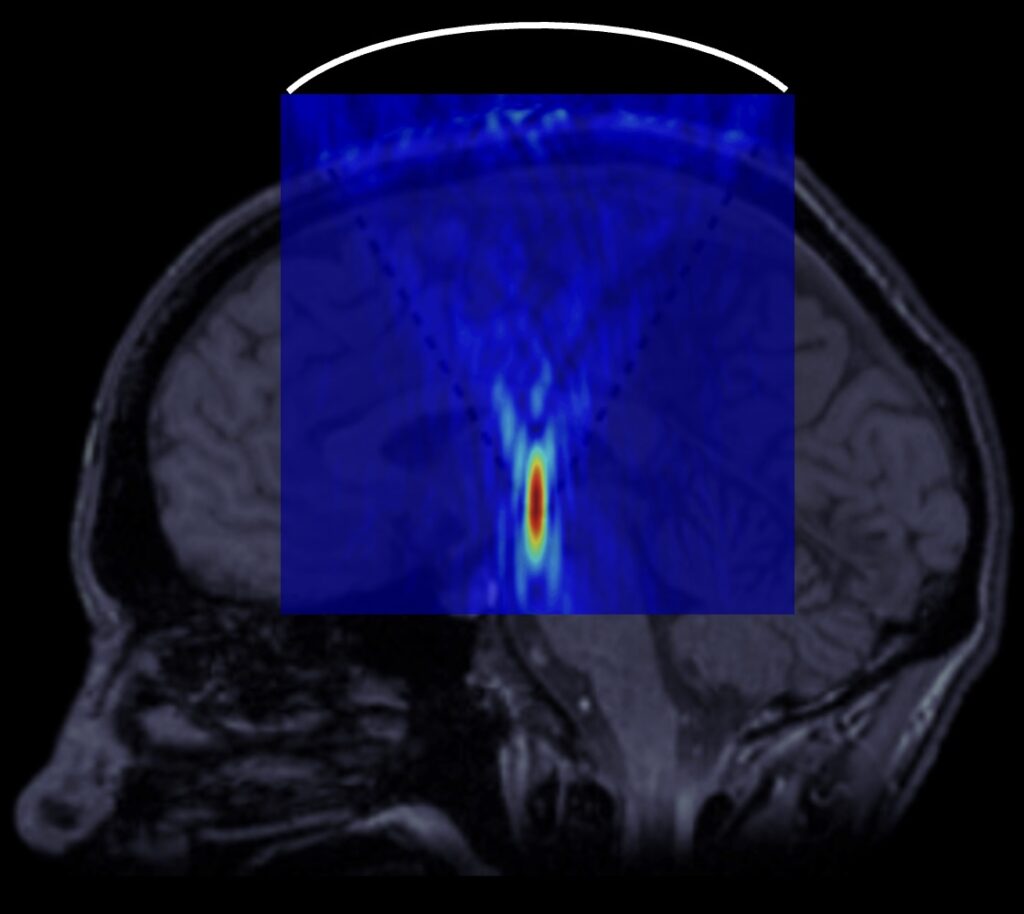
A diagram illustrating the performance of neurosurgical operations using high-intensity focused ultrasound. A focusing transducer is located outside the patient’s head directing the acoustic beam to a specified area of the brain.
The boiling histotripsy method developed in our laboratory uses a sequence of powerful short pulses of focused ultrasound with shock fronts at the focus that cause the formation of vapor cavities and cavitation microbubbles in the focal region. Their interaction with the shock fronts of the ultrasound wave leads to mechanical fractionation (liquefaction) of tissue into subcellular fragments. The non-thermal mechanism of such an effect can minimize the limitations of existing thermal HIFU methods and expand the scope of clinical indications for focused ultrasound-based treatment. Moreover, our method allows monitoring the process of exposure using conventional diagnostic ultrasound: vapor-filled inclusions in tissue during boiling histotripsy appear bright on ultrasound, whereas liquefied biotissue post-treatment appears dark.
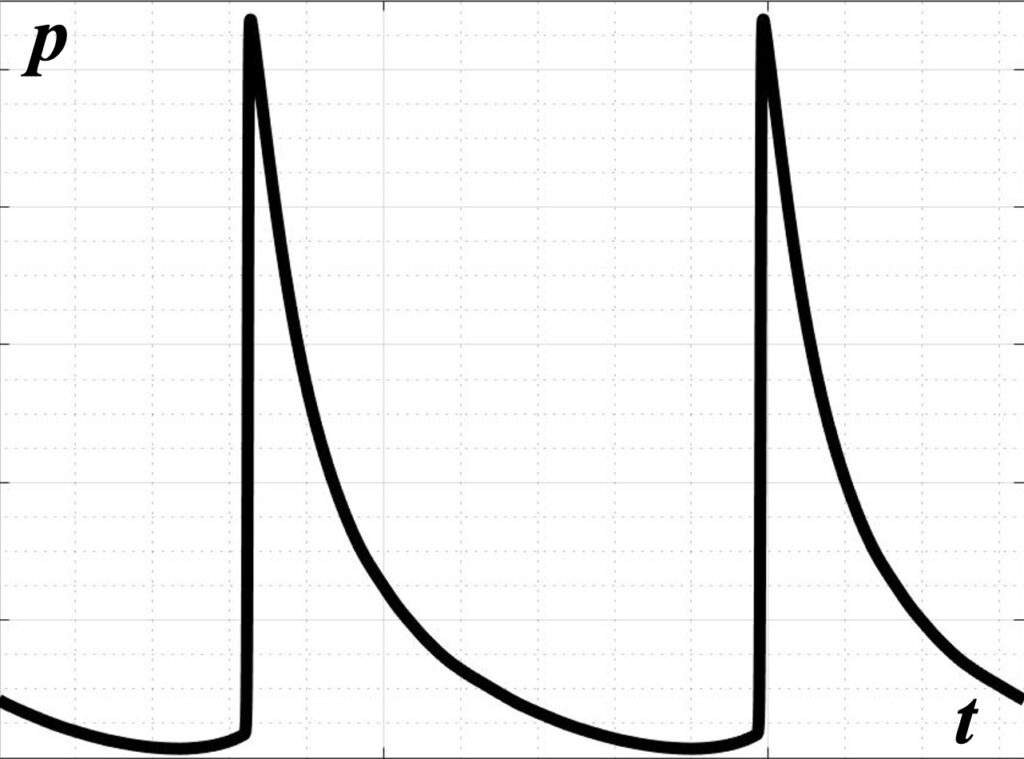
In high-amplitude pulse-periodic sanitation regimes, shock fronts are formed at the beam focus due to the effects of acoustic nonlinearity in the wave profile, which then lead to mechanical fractionation of the tissue.
Numerical simulations previously conducted at LIMU have already demonstrated the possibility to achieve the conditions and ensure safe use of boiling histotripsy with transcranial focusing of ultrasound in brain tissue, including close to the skull. The fundamental possibility of ultrasound-based control of the procedure by recording acoustic signals from the focus during the collapse of cavitation bubbles has also been demonstrated. However, the feasibility of boiling histotripsy for human brain ablation has not been investigated experimentally yet.
Therefore, in our recent joined studies with the Moscow State University Clinic, the Pulmonology Research Institute and the Human Morphology Research Institute, the first experiments were conducted demonstrating the fundamental possibility to generate mechanical lesions in various tissues of the human brain, to begin with, ex vivo (i.e., outside the body) and in the absence of the skull. Mechanical lesions were also successfully obtained in thalamus and globus pallidus – those parts of the brain that are currently being targeted in clinical treatment of movement disorders with thermal HIFU under MRI control.
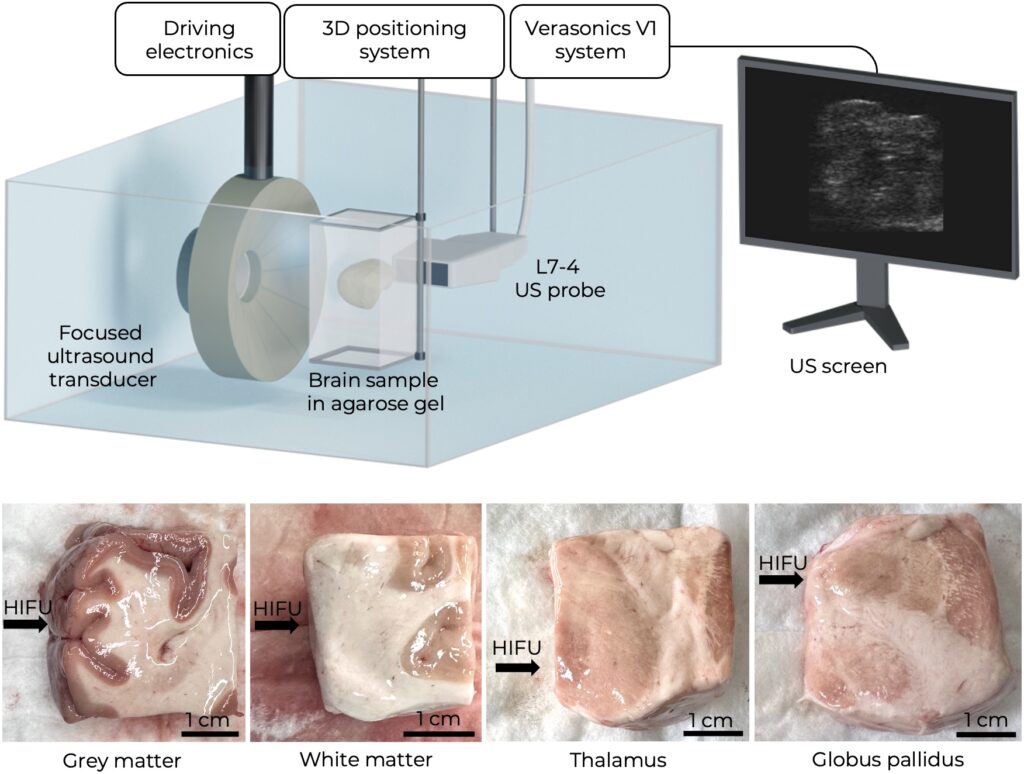
All experiments are accompanied by preliminary measurements of the tissue stiffness, since their susceptibility to histotripsy is mainly determined by their elastic properties. This is the reason why diagnostic methods of elastography (i.e., measuring tissue elasticity using ultrasound) actively help in the development of the boiling histotripsy method.
The obtained results confirmed the potential for expanding the range of diseases treatable with HIFU. Further research at LIMU is now aimed at experimentally confirming the feasibility of the method in the presence of bone tissue in the path of the ultrasound beam to bring the laboratory experiment closer to clinical conditions. If the method is successfully implemented in clinical practice, it will reduce the number of open surgeries on the brain, as well as the cost of such interventions due to the possibility of incisionless fractionation of target areas of the brain under conventional ultrasound control.

Contacts
Details
[1] Boiling histotripsy in ex vivo human brain: proof-of-concept / E. Ponomarchuk, S. Tsysar, A. Kadrev et al. // Ultrasound in Medicine and Biology. — 2025. — Vol. 51, no. 2. — P. 312–320. DOI: 10.1016/j.ultrasmedbio.2024.10.006
[2] Simulation of nonlinear trans-skull focusing and formation of shocks in brain using a fully populated ultrasound array with aberration correction / P. B. Rosnitskiy, P. V. Yuldashev, O. A. Sapozhnikov et al. // Journal of the Acoustical Society of America. — 2019. — Vol. 146, no. 3. — P. 1786–1798. DOI: 10.1121/1.5126685
[3] The histotripsy spectrum: differences and similarities in techniques and instrumentation / R.P. Williams, J.C. Simon, V.A. Khokhlova, O.A. Sapozhnikov, T.D. Khokhlova // International Journal of Hyperthermia, 40 1 1-19. DOI: 10.1080/02656736.2023.2233720
[3] Nonlinear acoustics today / O. A. Sapozhnikov, V. A. Khokhlova, R. O. Cleveland et al. // Acoustics today. — 2019. — Vol. 15, no. 3. — P. 55–64. DOI: 10.1121/AT.2019.15.3.55
[4] Shock-induced heating and millisecond boiling in gels and tissue due to high intensity focused ultrasound / M. S. Canney, V. A. Khokhlova, O. V. Bessonova et al. // Ultrasound in Medicine and Biology. — 2010. — Vol. 36, no. 2. — P. 250–267. DOI: 10.1016/j.ultrasmedbio.2009.09.010
[5] Controlled tissue emulsification produced by high intensity focused ultrasound shock waves and millisecond boiling / T. D. Khokhlova, M. S. Canney, V. A. Khokhlova et al. // Journal of the Acoustical Society of America. — 2011. — Vol. 130, no. 5. — P. 3498–3510. DOI: 10.1121/1.3626152
[6] Compensation for aberrations when focusing ultrasound through the skull based on CT and MRI data / D. D. Chupova, P. B. Rosnitskiy, O. V. Solontsov et al. // Acoustical Physics. — 2024. — Vol. 70, no. 2. — P. 288–298. DOI: 10.1134/s1063771024601651
[7] Compensation for aberrations of focused ultrasound beams in transcranial sonications of brain at different depths / D. D. Chupova, P. B. Rosnitskiy, L. R. Gavrilov, V. A. Khokhlova // Acoustical Physics. — 2022. — Vol. 68, no. 1. — P. 1–10. DOI: 10.1134/S1063771022010018
[8] A comparative study of experimental and simulated ultrasound beam propagation through cranial bones / A. Krokhmal, I. C.Simcock, B. E. Treeby, E. A. Martin // Physics in Medicine and Biology. — 2025. — Vol. 15, no. 70(2) — P. 025007. doi: 10.1088/1361-6560/ada19d
[9] Estimation of the thickness profile of a human skull phantom by ultrasound methods using a two-dimensional array / S. A. Asfandiyarov, P. B. Rosnitskiy, S. A. Tsysar et al. // Acoustical Physics. — 2023. — Vol. 69. — P. 112–118. DOI: 10.1134/S106377102270004X
[10] Use of pulse-echo ultrasound imaging in transcranial diagnostics of brain structures / D. A. Sukhoruchkin, P. V. Yuldashev, S. A. Tsysar et al. // Bulletin of the Russian Academy of Sciences: Physics. — 2018. — Vol. 82, no. 5. — P. 507–511. DOI: 10.3103/S1062873818050283
[11] On the possibility of using multi-element phased arrays for shock-wave action on deep brain structures / P. Rosnitskiy, L. Gavrilov, P. Yuldashev et al. // Acoustical Physics. — 2017. — Vol. 63, no. 5. — P. 531–541. DOI: 10.1134/S1063771017050104
[12] A multi-element interstitial ultrasound applicator for the thermal therapy of brain tumors / M. Canney, F. Chavrier, S. Tsysar et al. // Journal of the Acoustical Society of America, 134 2 1647–1655. DOI: 10.1121/1.4812883